Optimized bacterial DNA isolation method for microbiome analysis of human tissues (MicrobiologyOpen)
Optimized bacterial DNA isolation method for microbiome analysis of human tissues
Carlijn E. Bruggeling, Daniel R. Garza, Soumia Achouiti, Wouter Mes, Bas E. Dutilh, Annemarie Boleij
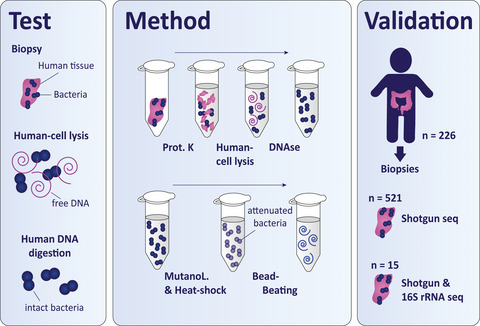
Recent advances in microbiome sequencing have rendered new insights into the role of the microbiome in human health with potential clinical implications. Unfortunately, the presence of host DNA in tissue isolates has hampered the analysis of host-associated bacteria. Here, we present a DNA isolation protocol for tissue, optimized on biopsies from resected human colons (~2–5 mm in size), which includes reduction of human DNA without distortion of relative bacterial abundance at the phylum level. We evaluated which concentrations of Triton and saponin lyse human cells and leave bacterial cells intact, in combination with DNAse treatment to deplete released human DNA. Saponin at a concentration of 0.0125% in PBS lysed host cells, resulting in a 4.5-fold enrichment of bacterial DNA while preserving the relative abundance of Firmicutes, Bacteroidetes, γ-Proteobacteria, and Actinobacteria assessed by qPCR. Our optimized protocol was validated in the setting of two large clinical studies on 521 in vivo acquired colon biopsies of 226 patients using shotgun metagenomics. The resulting bacterial profiles exhibited alpha and beta diversities that are similar to the diversities found by 16S rRNA amplicon sequencing. A direct comparison between shotgun metagenomics and 16S rRNA amplicon sequencing of 15 forceps tissue biopsies showed similar bacterial profiles and a similar Shannon diversity index between the sequencing methods. Hereby, we present the first protocol for enriching bacterial DNA from tissue biopsies that allows efficient isolation of all bacteria. Our protocol facilitates analysis of a wide spectrum of bacteria of clinical tissue samples improving their applicability for microbiome research.